Antibody C-Terminal Variation Analysis Service
Antibody variations are minor structural modifications within antibody molecules that potentially impact their functionality and stability. Notably, variations often occur at the C-terminal—the heavy chain's end—of the antibody. These can arise from RNA editing errors during genetic recombination, post-translational modifications, or both internal and external cellular factors. Common C-terminal variations include incomplete cleavage, amino acid substitutions, and extensions of glycine. Such changes predominantly take place during antibody synthesis and post-translational modifications in cellular or secretory processes. For instance, the specificity and efficiency of enzymatic recognition can lead to non-specific cleavages at the antibody's C-terminal. These alterations may compromise the structural integrity and functional activity of the antibodies, diminishing their affinity, altering immunogenic properties, or affecting their half-life. Moreover, such variations can influence the purity and yield during antibody production, thereby impacting the biopharmaceutical's safety and efficacy. Conducting detailed analyses of C-terminal antibody variations is critical for maintaining biopharmaceutical quality and therapeutic effectiveness. High-precision mass spectrometry, combined with bioinformatics tools, serves as the cornerstone for identifying and quantifying these variations accurately.
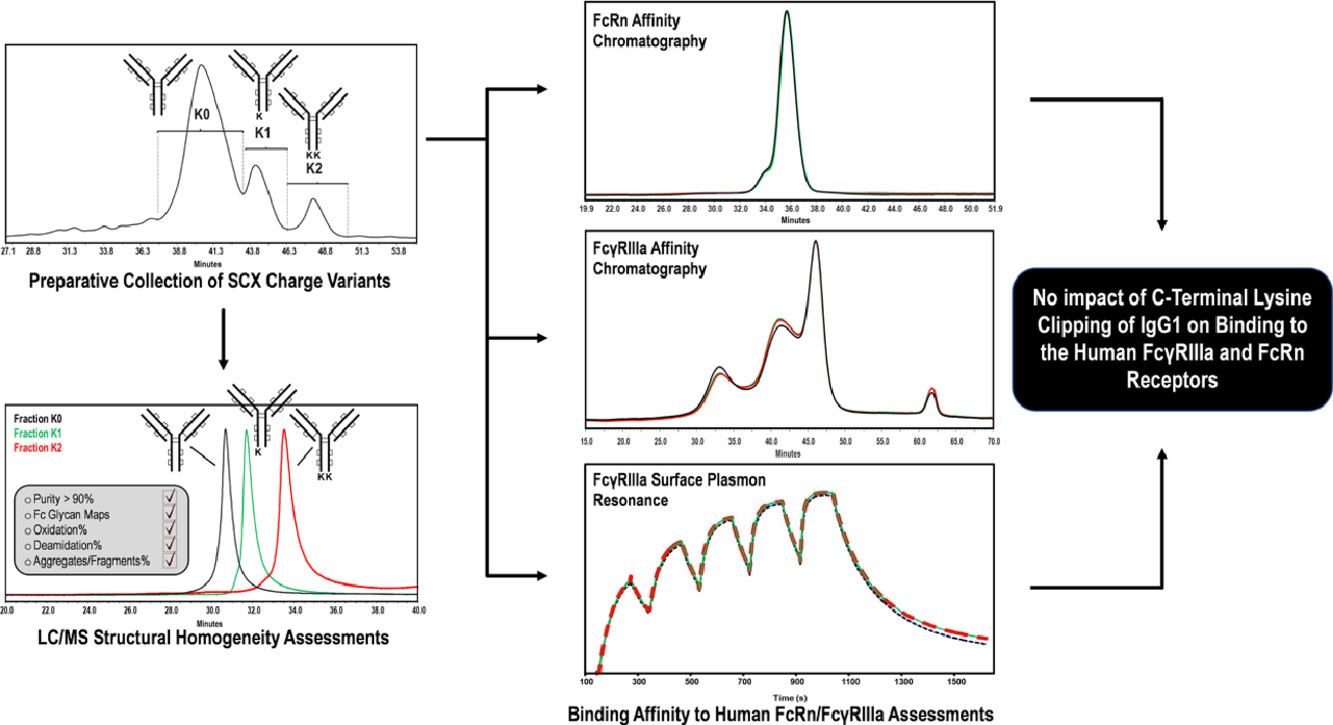
Faid, V. et al. Eur J Pharm Sci. 2021.
Figure 1. C-terminal Lysine Clipping of IgG1
Services at MtoZ Biolabs
With extensive expertise in analyzing antibody C-terminal variations, MtoZ Biolabs has developed a robust antibody C-terminal variations analysis platform featuring state-of-the-art mass spectrometers like the Thermo Fisher Q ExactiveHF and the Orbitrap Fusion Lumos, integrated with a Nano-LC system. We also extend our analytical capabilities to N-terminal variation analysis of antibodies, aiming to offer exhaustive research support. Free project evaluation, welcome to learn more details! Our technical specialists are available to provide a free business assessment.
Analysis Workflow
1. Sample Preparation: Extracting and purifying antibodies.
2. Enzymatic Digestion: Antibodies are digested using specific enzymes to release C-terminal fragments.
3. Mass Spectrometry Analysis: Samples are analysed by high-resolution mass spectrometry to identify C-terminal variations.
4. Data Analysis: Data is processed and analyzed using sophisticated software, providing actionable insights and recommendations.
Service Advantages
1. High Precision Identification: Our advanced mass spectrometry ensures accurate and reproducible detection of variations.
2. Quantitative Analysis: Our service extends beyond identifying variation types to offer quantitative insights, enriching data support.
3. Expert Interpretation: Analyses are interpreted by seasoned bioinformaticians and biochemists.
4. Broad Applications: Our service caters to multiple sectors, including drug development, preclinical research, and quality control.
Applications
1. Drug Development and Optimization: Ensuring high purity and consistency in antibody drugs is paramount during their development. Analyzing C-terminal variations allows teams to refine antibody production, boosting drug stability and effectiveness.
2. Quality Control: C-terminal variation analysis is vital for controlling product consistency across and within production batches, guaranteeing adherence to stringent quality standards.
3. Clinical Research: In clinical trials, C-terminal variation analysis helps assess how antibody drugs behave in the human body, crucial for determining their safety and efficacy.
4. Disease Diagnosis: Some techniques for analyzing C-terminal variations are also employed in diagnosing and monitoring diseases, aiding in more accurate disease identification and management.
Deliverables
1. Experimental Procedures
2. Relevant Mass Spectrometry Parameters
3. Detailed Information on Antibody C-Terminal Variation Analysis
4. Mass Spectrometry Images
5. Raw Data
How to order?







